Following are names & locations of Pharmaceuticals Companies sealed in Pakistan during last three years on account of manufacturing of spurious medicines;
- Everest Pharmaceuticals (Pvt) Limited
Industrial Triangle, Kahuta Road, Islamabad
- Ambro Pharma (Pvt) Limited
Industrial Triangle, Kahuta Road, Islamabad
- Royal Herbal Enterprises Company
Office Baldia Town No. 3 Karachi
Also Read: List of medicines declared spurious in Pakistan
Complaints received from Pharmaceuticals Firms regarding similarity of brand names;
There are approximately 65,000 human medicines registered so far with the Drug Regulatory Authority of Pakistan (DRAP) and in last six months, a total of 61 cases of look-alike/sound-alike brand of medicines i.e. identical packaging designs and color schemes etc were reported to the DRAP.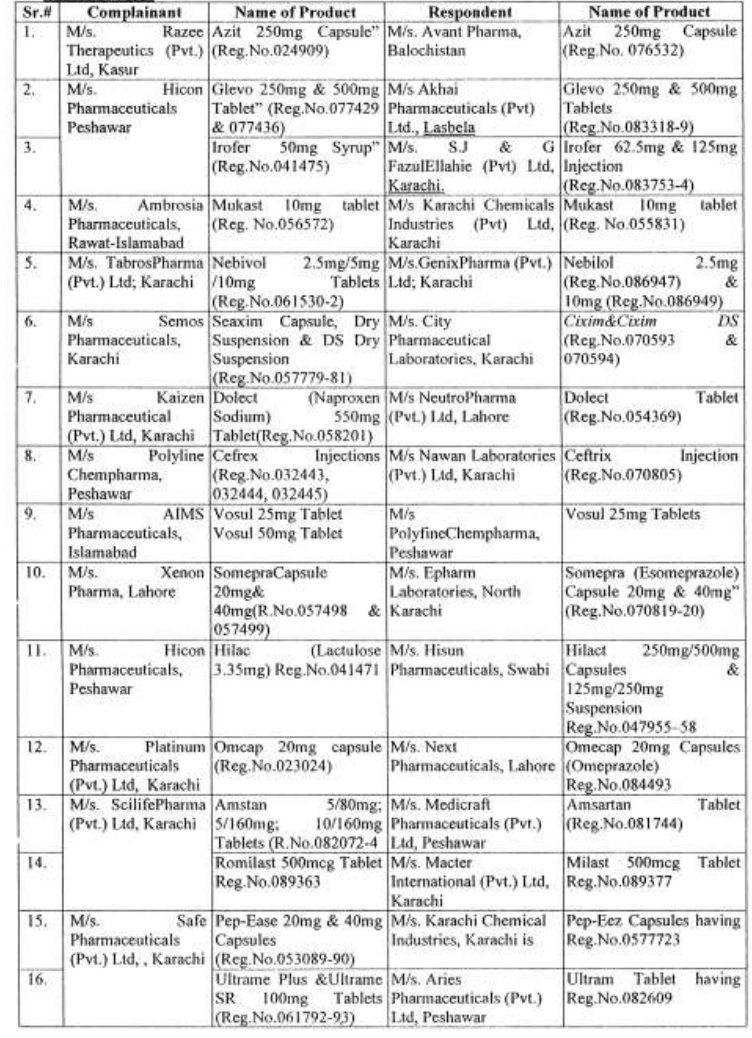
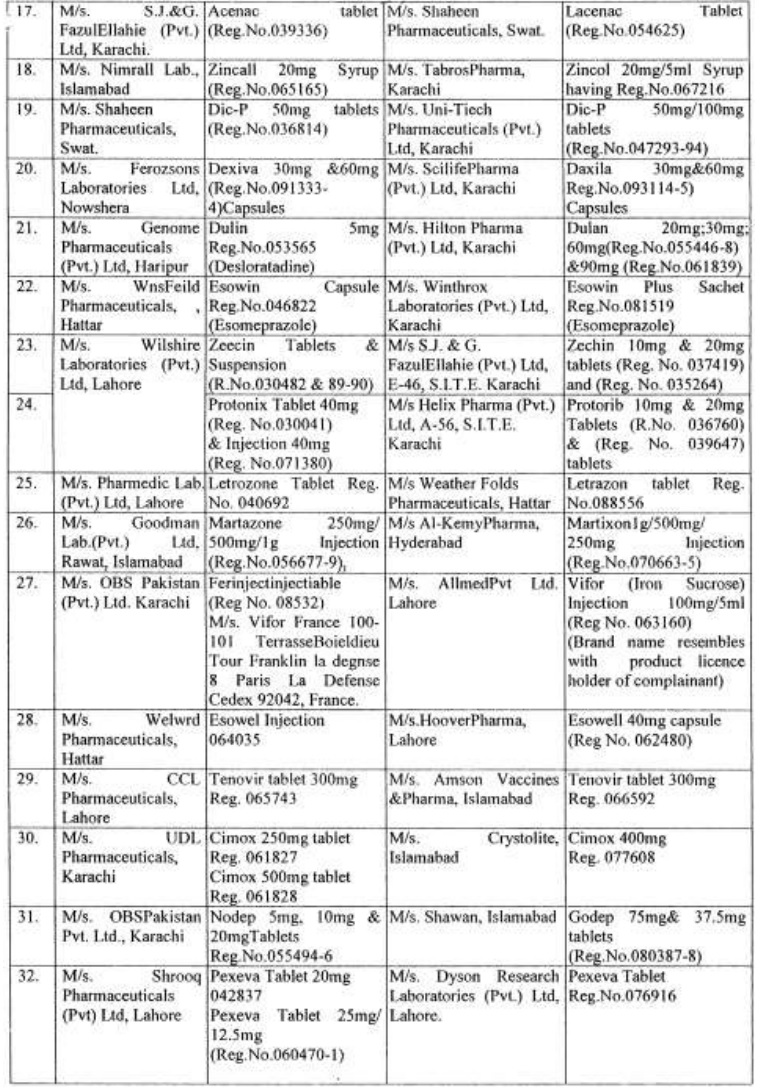
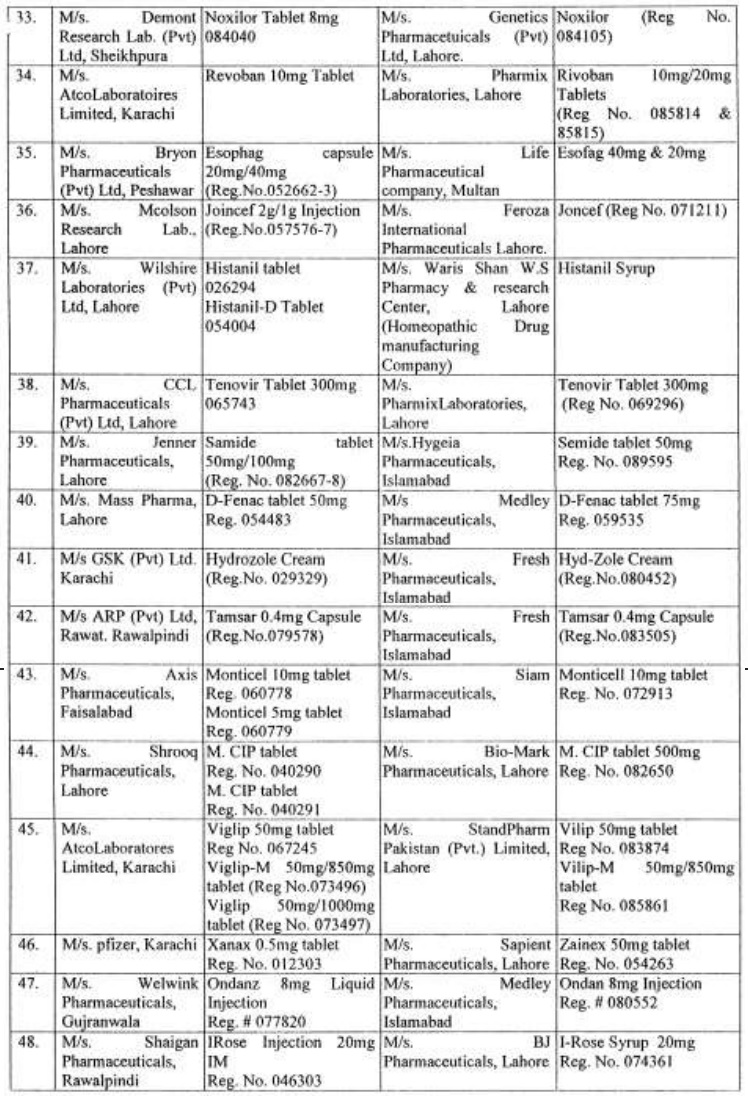
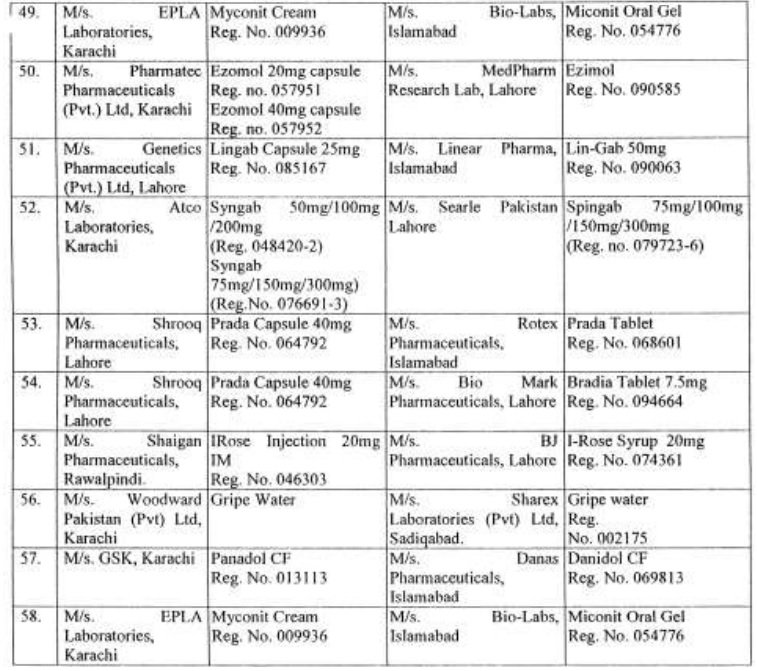
Complaints received from Pharmaceuticals Firms regarding similarity of packing material;
Procedure for establishment of Pharmaceutical Unit
As many as 74 Licenses have been granted for manufacturing of drugs/medicines to various manufactures in the Country during the last four years.
Procedure for grant of a Drug Manufacturing License under Drug Act 1976;
- Receipt of application for site verification
- Verification of suitability of site by panel of inspectors for establishment of Pharmaceutical Unit
- Approval of site for establishment of Pharmaceutical Unit
- Receipt of Layout Plan along with prescribed fee
- Scrutiny and Approval of Layout Plan(s)
- Construction of Factory Premises by applicant
- Receipt of application for grant of License on Form-I along with requisite document & fee
- Plan Inspection before grant of Drug Manufacturing License (DMD)
- Consideration of application by Central Licensing Board (CLB) in the light of recommendation of panel.
- Approval for grant of Drug Manufacturing License (DMD) by Central Licensing Board (CLB)
- Issuance of Drug Manufacturing License (DMD) for a period of five years and subsequent renewal for further period of five years
Note: The above data/information was shared by the Minister for National Health Services, Regulations and Coordination with the Senate on January 14, 2020.
